Things to keep in mind as you sample
To pass Australia’s strict quarantine laws:
Your leaf or seed samples MUST be pulverized to ensure that they are not turned away, held at customs and/or sent back to you. We recommend using a single 3mm stainless steel ball bearing in each tube such as https://us.vwr.com/store/product?keyword=76533-062
Do not send undried tissue. Samples can be older, or freshly collected, but must be dried and pulverized prior to shipping. Undried tissue is not permitted into Australia from the US, and the likelihood that samples will mold/become contaminated in transit is very high.
Do not include any of drying agents in your shipping container. They could be considered hazardous materials and denied at customs.
For the best samples:
You can collect samples on ice blocks or flash freeze in liquid nitrogen, and dry them using a desiccant like silica beads or gel.
Oven drying is not recommended.
For successful orders:
Do not mix different tissue types (e.g. leaves and roots) or species in the same plate.
You must create separate orders for each genus.
Please ensure you have equal and consistent size samples per the guidelines.
If samples are not consistent, DArT may need to sub-sample manually, and this may affect the time it takes to process your order.
Plasticware Recommendations
Before you begin sampling, be sure you are using DArT-approved plasticware. The same plasticware can be used for leaf tissue or pulverized seeds. Do not mix different types of samples in your project order.
If you have already begun sampling using different plates, make sure to transfer to DArT-approved plates before shipping. Failure to send samples in correct plasticware will greatly affect the timeline of your project.
If you have difficulty ordering or obtaining these products in a timely manner, please contact Breeding Insight at bi-genotyping@cornell.edu . We may have some plasticware in stock that can be shipped quickly.
Plasticware for Tissue or Pulverized Seed Samples
Sending Pulverized Seed Samples
About 70-90mg of pulverized seeds will need to be sampled into each well of an approved Microtube Rack System.
Take care to avoid cross-contamination between samples.
Label the tubes with their well position within the plate.
Leave tubes G12 and H12 empty, as these are used for control purposes.
Sending Leaf Samples- 2 Methods
**Keep in mind that regardless of the method you choose to sample your leaf tissue, your final samples must be dried and pulverized.**
You need to provide 10-15mg of dried plant tissue per tube. Depending on your sample type, there are two recommended methods for cutting leaves.
Method 1 – Tube Cutting Leaf Samples
This method is suitable for most leaves excluding acicular or linear leaves. With this method, you can easily cut leaves into equal size 3-5mm discs by:
1.Folding the leaf twice
2.Using the open end of the collection tube as a stencil to cut the discs
3.Pushing the disc down into the tube, add enough discs to get 10-15mg of dried tissue.
Leave tubes G12 and H12 empty as these are used for control purposes.

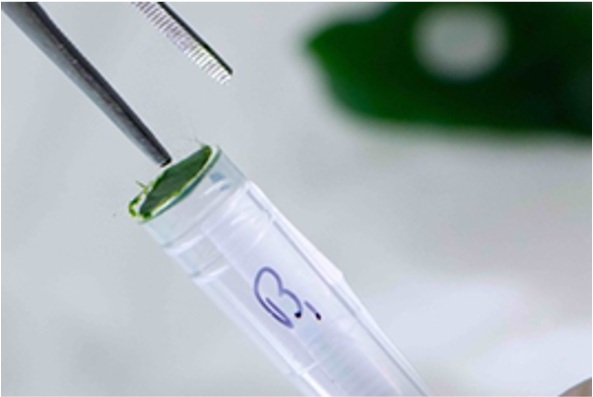
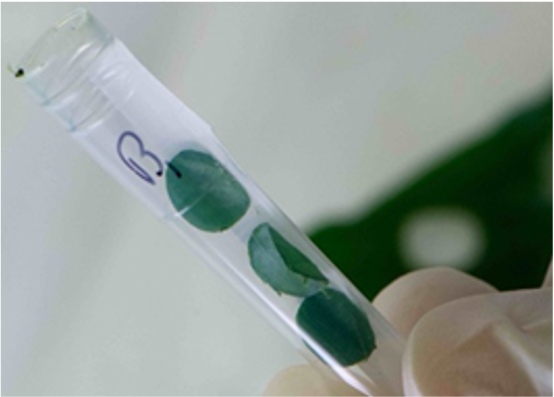
Method 2 – Manually Cutting Leaf Samples
You can also manually cut leaves into 5-10mm pieces. Ensure that the sample sizes are uniform and consistent across all tubes. This ensures the leaves will be pulverized efficiently for successful DNA extraction.
Cut the leaves with scissors or a blade, wiped clean with 70% ethanol between samples, to avoid cross contamination.
Leave tubes G12 and H12 empty as these are used for control purposes.
Do this.

Not this.





